All surfactants have molecular structures that include both hydrophilic (water-loving) and hydrophobic (water-hating) parts. It is this feature which imparts surface activity to surfactants. When added to water, the hydrophilic parts are easily dissolved in the water, whereas the hydrophobic parts are water insoluble. This results in the surfactant orienting itself at the interface between the water and the vessel holding the water, as well as the surface between the water and the air. In each case the hydrophilic part of the surfactant oriented towards the water and the hydrophobic part of the surfactant oriented away from the water.

Consider a drop of water on a freshly waxed surface. The surface is very hydrophobic and water cannot easily wet or spread over the surface. The water tends to associate with itself rather than the waxy surface, forming a bead on the waxy surface. If surfactant is added to the drop of water, the surfactant would migrate to the surface of the water drop, where the hydrophobic part of the surfactant molecule can easily associate with the waxy surface. Thus, the surface tension is lowered and the water bead begins to spread and wet out more of the waxy surface.
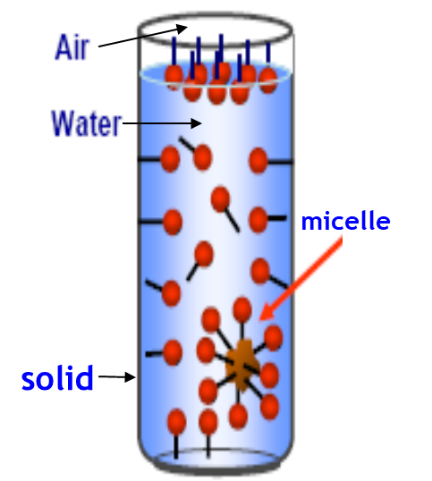
This ability to reduce surface tension is a key measure of surfactant performance. The amount by which surface tension may be reduced is dependent upon several factors including the amount of surfactant which can exist at the water surface, referred to as the packing density. When a low level of surfactant is added to water some of the surfactant will dissolve in the water and most will migrate to the water surface. As more and more surfactant is added to the mixture, the solution and the surfaces both become saturated. When this happens, additional added surfactant forms soluble aggregates with their hydrophilic parts oriented outward, toward the water, and their hydrophobic parts oriented inward, away from the water. These aggregates are called micelles. The concentration at which micelles start to form is called the critical micelle concentration or CMC. The surface tension for a given surfactant is minimized at or above the CMC, meaning no more surfactant can be accommodated at the surface and, thus, no further reduction in surface tension will occur.
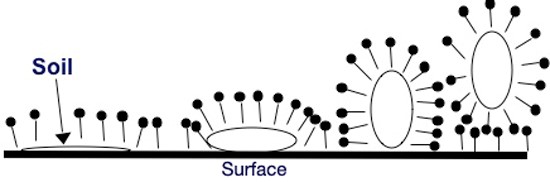
Surfactants work by attaching their hydrophobic portions to an oily soil on the surface. The surfactants then work between the oily soil and the surface, effectively increasing the available surface area for surfactant. This process, which ultimately removes the oily soil deposit from the surface, is known as oily soil roll up and is represented in the figure below. Individual surfactant molecules are represented by the pin shape, where the solid circle represents the hydrophilic head group and the straight line represents the hydrophobic tail group. The oily soil deposit is represented by the open circle.
Surfactants are commonly classified or grouped based on the electrical charge of their hydrophilic head groups. Accordingly, surfactants are classified as anionic (the head group contains a negative charge), cationic (the head group contains a positive charge), nonionic (the head group does not contain a charge), and amphoteric (the head group contains both negative and positive charges depending on the pH). The charge on a surfactant will give it specific properties and functionality.
Anionic surfactants, with an overall negative charge, account for the largest class of surfactants in terms of volume surfactant used. Widely used anionic surfactants include sulfonates, sulfates, ether sulfates and carboxylates. Anionic surfactants are the major surfactant in most hard surface cleaners, including manual dish wash products, all-purpose cleaners, floor cleaners, bathroom tub and tile cleaners, and a whole range of specialty hard surface cleaners. In general, anionic surfactants are high foaming products, although there are exceptions.
Cationic surfactants, especially those with longer hydrophobic tails, tend to be very substantive on surfaces. Because most surfaces carry a negative charge, the positively charged cationic surfactants have a high attraction to the negatively charged surface. Accordingly, these surfactants are used as hair and fabric conditioners adding softness or shine to these surfaces when attached. Cationic surfactants, with shorter hydrophobic tails, often demonstrate biocidal properties and are used as the active ingredient in many disinfectant and sanitizer products.
Nonionic surfactants include alcohol alkoxylates, alkylphenol ethoxylates, ethylene oxide propylene oxide block co-polymers, fatty acid amides, fatty acid esters, and alkyl glucosides. In general, nonionic surfactants are good degreasers and create medium to low foaming products. Nonionic surfactants can be used in various applications where high foam is not desirable or in automatic dishwashing where foam is a detriment to good cleaning. They can be used in combination with any other surfactant types (both cationic and anionic), since nonionic surfactants do not have a charge to react with the charge of another surfactant type. Combining surfactant types can result in increased cleaning performance.
Amphoteric surfactants, often called zwitterionic surfactants, tend to form stable foam and, as such, are often included as a co-surfactant in products where a stable foam is desired. A amphoteric substance can be anionic or cationic depending on the pH. Under acidic conditions, these becomes cationic and at alkaline pHs they become anionic in nature. At a neutral pH near 7, amphoteric surfactants are relatively mild towards skin, hence they commonly used in personal care products such as shampoos and body wash products. Commonly used amphoteric surfactants include betaines, amphoacetates, amphopropionates, and amine oxides.
While the nature of the polar head group is highly variable and determines many of the overall characteristics of the surfactant, it is the balance between the head group and the hydrophobic tail group that gives surfactants their surface activity. Hydrophobic tail groups fall into three broad categories: linear alkyl chains, aryl ring structures (often with linear alkyl chains attached), and propylene oxide oligimers. The larger the hydrophobic group, the less water soluble the surfactant and the higher the viscosity of the solution. The reduced water solubility leads to a lower critical micelle concentration and more efficient surface tension reduction.
Surfactant structures with short chain hydrophobes, for example xylene sulfonate and cumene sulfonate, have very little surfactant character. Despite this, these materials are very useful as aids in solubilizing surfactants with relatively poor water solubility. These materials are called hydrotropes.
For a more detailed discussion of surfactants, their structures and their properties we refer the interested reader to the Surfactant Science Series of text books published by Marcel Dekker Inc. This series of textbooks contains over 70 volumes discussing all aspects of surfactant science.
