
A chelant, sometimes referred to as a sequestrant or a builder, is a specialized molecule designed to bind to positively charged metal ions, most commonly calcium and magnesium, in solution. This coupling prevents the metal ions from forming insoluble precipitates with other ions that may be present in solution. The two basic types of chelants are designated as hard and soft.
Hard chelants example EDTA (ethylenediamine tetra acetic acid) and NTA (Nitrilo triacetic acid) form very stable complexes with a 1:1 stoichiometry. That is to say that one EDTA molecule reacts with a single metal ion.
Soft chelants like polymers of acrylic and maleic acid form less stable complexes and function by preventing crystal growth rather than by true chelation.
Both calcium and magnesium ions form very stable insoluble precipitates with carbonate ion. Bicarbonate is ubiquitous in surface waters and is deprotonated by high pH cleaners to give carbonate. Insoluble precipitates are difficult to remove without the use of an acidic cleaner. These difficult to remove deposits are called hard water scale, and thus water containing calcium and magnesium ions is referred to as hard water. There are two common methods to deal with hard water. The first is to use softened water. Softened water has the calcium and magnesium ions removed. This is often accomplished by use of a water softener which exchanges sodium ions for the calcium and magnesium ions in solution. The second method is to use chelants in the cleaning product formulation. Chelating the calcium and magnesium ions in solution effectively prevents the formation of these deposits.
Calcium and magnesium ions also form insoluble precipitates with many anionic surfactants, most notably fatty acid carboxylates (soap). The formation of calcium soap complexes is so efficient that the concentration of free surfactant available for cleaning is reduced to effectively zero until all the calcium has been removed from solution either by chelation or by forming the insoluble soap adduct. Without the chelant present in a cleaning product more surfactant would be necessary to ensure that an effective concentration of free surfactant was available for cleaning. In other words, the consumer would have to use more of the cleaning product to obtain a desired result. Further, the insoluble calcium soap adduct would precipitate from solution onto the surface to be cleaned leading to a dingy appearance and a stiff feel on the washed fabric. Thus, chelants used for these purposes are sometimes referred to as “builders” as they help build upon the performance of the surfactant.
In addition to softening the water of the cleaning solution, chelants are also effective in helping to remove some stains, especially those that have a high metal ion concentration. Stains have a complex and varied molecular structure that often include metal ions cross linking and stabilizing the stain structure. Chelants present in the cleaning solution will bind with these various metal ions helping to remove them from the stain and thereby weakening the stain structure to facilitate its removal.
Beyond calcium and magnesium, chelants bind with all polyvalent metal ions in solution. Iron and manganese, although less prevalent, can also lead to significant cleaning problems including the formation of insoluble precipitates such as iron oxide, rust, and manganese dioxide. Therefore, specialty cleaners are often formulated with highly efficient iron chelators to bind with these ions, again eliminating the problems they might otherwise cause or helping to eliminate rust and iron stains that have already formed.
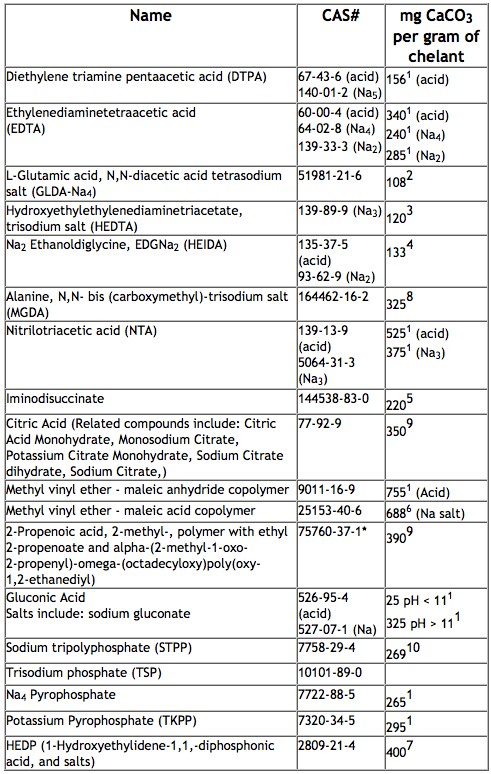
The table below shows many examples of chelating agents used in formulated products and their theoretical capacity to bind with or sequester calcium from water to improve cleaning performance:
Resources:
1. Synthetic Detergents Seventh Edition, AS Davidsohn & B Milwidsky, Longman Scientific & Technical, 1987
2. Akzo Nobel Dissolvine GL brochure, March 2004.
3. Dow Versenol 120 Chelating Technical Datasheet, June 2002.
4. Dow Versene HEIDA Chelating Agent Technical Datasheet. Form No. 113-01355-04-07.
5. Bayer Baypure CX-100 Edition 2001-11
6. ISP Unpublished data.
7. http://www.kelien.com/products/HEDP_2809-21-4.htm
8. BASF Trilon Types Chelating Agent, June 2008
9. United States Patent 3904685
10. JAOCS 60 (3): 618-622, March 1983
